In today’s regulated industries, computerized systems play a central role in manufacturing, laboratory, and quality operations. To ensure data integrity, patient safety, and regulatory compliance, these systems must undergo rigorous Computer System Validation (CSV).
At SVU Enterprises, we specialize in delivering end-to-end CSV solutions that are fully aligned with international regulatory expectations, including 21 CFR Part 11, EU Annex 11, GAMP 5, WHO, and MHRA guidelines.
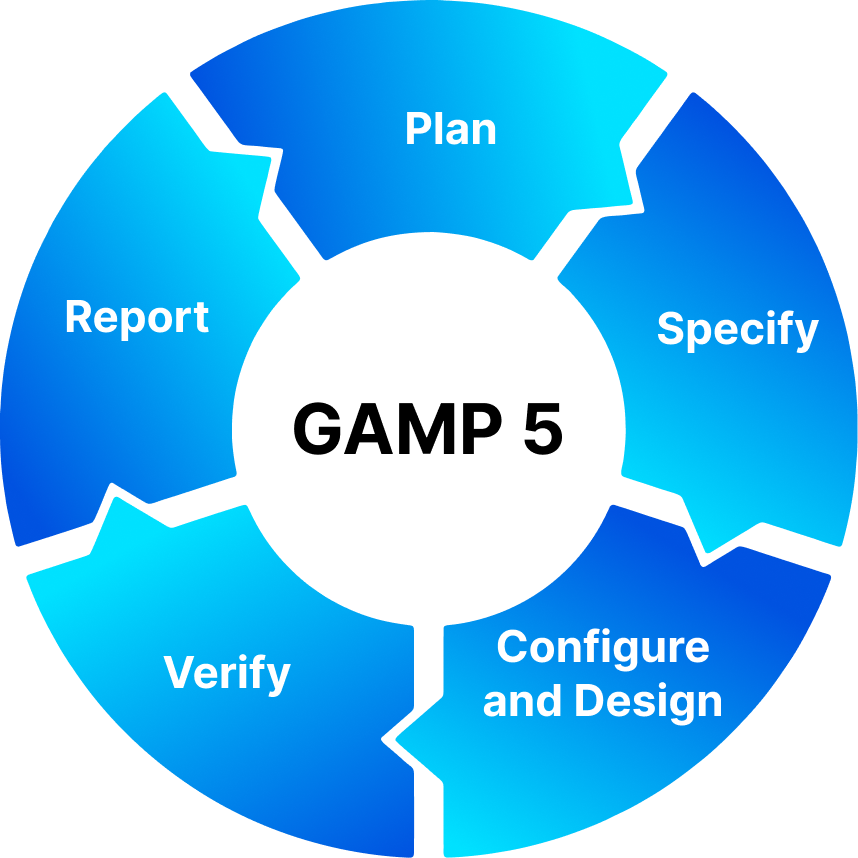
We follow the Good Automated Manufacturing Practice (GAMP 5) framework, which provides a structured and risk-based approach to validating computer systems. Our documentation covers the full system development lifecycle (SDLC):
By adopting the GAMP 5 model, we ensure a scalable, efficient, and risk-based validation process, reducing unnecessary testing while maintaining compliance.
For systems that handle electronic records and electronic signatures, compliance with USFDA 21 CFR Part 11 is critical. We provide:
This ensures your systems are ready for USFDA, EMA, and other global regulatory audits.
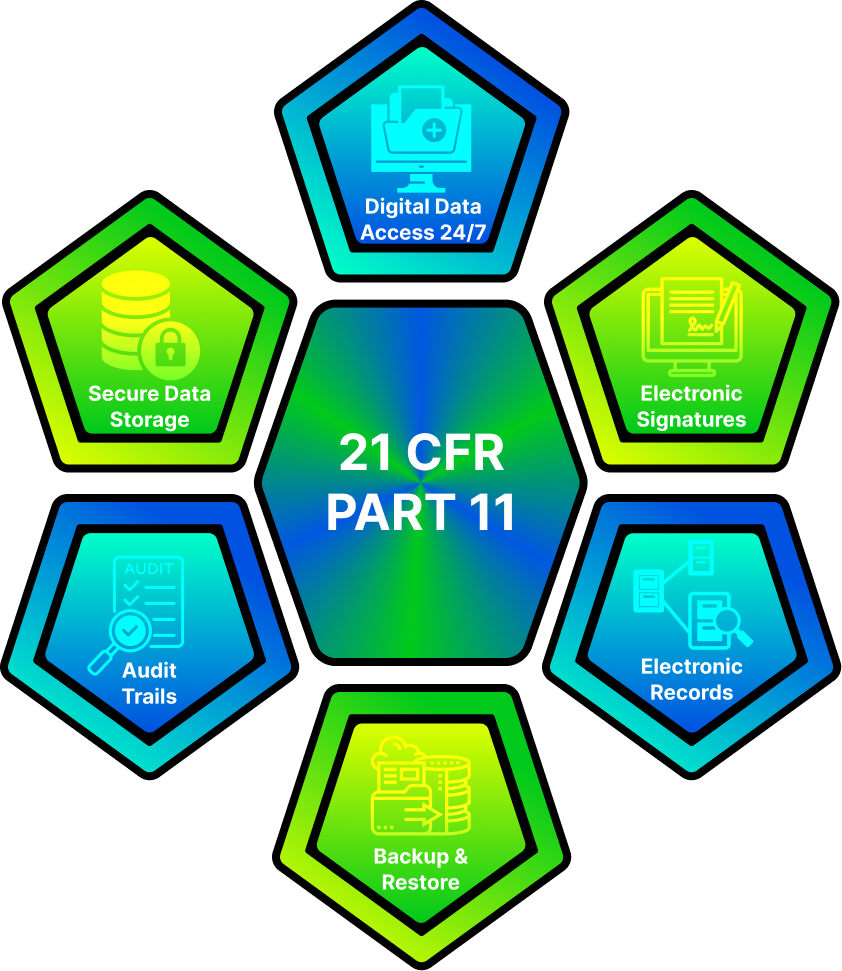

For systems that handle electronic records and electronic signatures, compliance with USFDA 21 CFR Part 11 is critical. We provide:
This ensures your systems are ready for USFDA, EMA, and other global regulatory audits.

Regulators expect well-structured documentation and clear evidence of validation execution. We prepare and execute validation deliverables that withstand the toughest audits, including:
With SVU Enterprises, you gain the confidence that your computerized systems are audit-ready at any time.